Humic substances are present in all organic ecosystems: oceans, rivers, lakes, and top soils. Quantifying the amount of humic material present in these systems is essential for academic research and industrial applications, specifically agricultural soil management. Additionally, testing humic acid levels is essential for companies, like Ocean Agro LLC, who are marketing humic acid based products in order to guarantee the amount of active ingredient present. Finally, understanding the amount of humic material present as a present of soil organic matter (SOM) is critical in developing a case for humic acid supplementation of specific soils and developing predictive indicators for product effectiveness.
Humic substances can be broken down into Humic Acid, Fulvic Acid, and Humin. Currently, humic substance testing has been centered on methods for quantifying Humic Acid content in soil and product samples, which will be the focus of this discussion. However, additional separation/quantification methodologies exist for fulvic acid (a highly soluble component of humic substances), and are currently being standardized through a collaboration between the International Humic Substances Society (IHSS) and Humic Products Trade Association (HPTA), which has resulted in the publication of A New Standardized Method for Quantification of Humic and Fulvic Acids in Humic Ores and Commercial Products. While the fundamentals of fulvic acid separation will be discussed here, this new testing standard for Fulvic Acid testing will be covered in greater detail as part of a future post.
Approaches for testing humic acids:
In order to test humic acid, one must first understand the generalized separation scheme for purifying humic substances into more soluble forms. Generally, all humic tests fall into three categories:
- Precipitation methods (standard approach for humic acid; principle focus of this post)
- Spectrophotometric methods (rapid, less accurate method; to be discussed in a future post)
- Ash content measurement methods (typically only used for measuring humic content; discussed in greater detail as part of our SOM testing procedure overview).
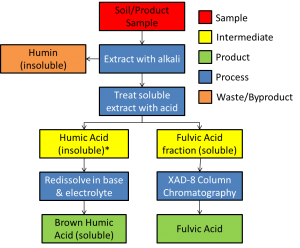
Most precipitation techniques for humic testing undergo critical alkaline (basic) and or acid treatments prior to centrifugation. While the specific bases and acids used can vary amongst different separation strategies, the approach of base then acid is the generally accepted standard. The figure below provided a comprehensive diagram of the generalized base/acid precipitation approach:
Table 1: Scheme for the isolation of humic substances from soil [Adapted from Stevenson (1994)]; *California Department of Food and Agriculture (CDFA) testing process end point (discussed below)
As mentioned above, humic acid testing presents the most developed standardized methodologies. First and foremost is the CDFA humic testing methodology, representing the current industry standard in California (making it arguably the most influential). However, other testing methodologies exist that report both higher and lower levels of humic acid for the same sample, with most reporting higher levels relative to the CDFA method (Lamar et. al 2009). The full CDFA humic acid testing protocol can be found here.
Among the other available precipitation methods for testing Humic Substances, several approaches exist that involve varying the levels and type of acids and bases used in the process: IHSS protocol, barium chloride extraction (resulting in higher reported values, Fataftah et. al ), and a classical extraction technique described by Schnitzer 1982 (included the repeated washing by Hydrochloric Acid (HCl)/Hydrogen Flouride (HF) solution to minimize ash content). All of these precipitation methods involved washing and removing non-humic material from the samples and drying and weighting humic acid precipitate.
As indicated in the figure above, Fulvic acid extraction and quantification involves a more complex chromatography step to concentrate the highly soluble fulvic acid solution found in the acid extraction supernate. A more complex discussion on fulvic acid extraction and quantification will be developed by our team in the near future.
As for the spectroscopy approaches mentioned initially, several techniques are available. Our SOM testing blog post discusses one such technique that involved a toxic chromic acid digestion step. Lamar et. al 2009 explores a novel colorimetric method that involves an alkaline extraction with dilute Sodium Hydroxide (NaOH) and Diethylene triamine pentaacetic acid (DTPA). One takes the solubilized solution (from alkaline extraction) and measures the amount of light that is able to pass through at a specific wave length, which can be used to infer humic acid concentrations using a reference standard. The main consideration with these methods is the representative standard used for calibration. While the conventional approach to build out a standard curve using a humic acid standard procured from the IHSS, it is also possible (and potentially more accurate, Lamar 2009) to develop a humic acid standard from the source material being tested. In the case of most commercial humic acid products, this would be leonardite ore. Regardless of the colorimetric (spectroscopy) approach used, the results typically reflect higher values relative to more standardized methods (Lamar 2009).
In conclusion, the testing method used can directly impact the accuracy of the test and thus the % of Humic substances present. While the optimal methodologies for extracting and quantifying humic material are still a work in progress, the large number of extraction processes available for creating purified humic substances creates a large amount of product variability within the humic acid market. Thus there exists a need for standardization of the testing and measuring protocols for Humic substances to ensure a ‘level playing field’. Consumers can buy with confidence as the % of HS present are same across different products (when measured with the same technique) and it also gives producers a way of directly competing – stimulating innovation.
References:
Aiken, G. R., and P. MacCarthy. “Isolation and Concentration Techniques for Aquatic Humic Substances.” Ed. D. M. Knight and R. L. Wershaw. Humic Substances in Soil, Sediment, and Water. Ed. G. R. Aiken. New York: Wiley, 1985. N. pag. Print.
Fataftah, A. K. “A Comparative Evaluation of Known Liquid Humic Acid Analysis Methods.” Humic Substances: Structures, Models, and Functions. Ed. Elham A. Ghabbour and Geoffrey Davies. N.p.: n.p., 2001. N. pag. Print.
Hayes, Michael H.B., Colin L. Graham, and Davies. “Procedures for the Isolation and Fractionation of Humic Substances.” Ed. Ghabbour. Humic Substances: Versatile Components of Plants, Soil and Water. N.p.: Royal Society of Chemistry, n.d. N. pag. Print.
Lamar, Richard, and Karen Talbot. “Critical Comparison of Humic Acid Test Methods.” Communications in Soil Science and Plant Analysis 40.15 (2009): 2309-322. Print.
Schnitzer, M. “Organic Matter Characterization.” Ed. R. H. Miller and D. R. Keeny. Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties. Ed. A. L. Page. Madison: American Society of Agronomy, 1982. 581-94. Print.
Stevenson, Frank J. Humus Chemistry: Genesis, Composition, Reactions. New York [etc.: J. Wiley, 1994. Print.
Tipping, Edward. Cation Binding by Humic Substances. New York: Cambridge UP, 2002. Print.